UK
Orabloc
Articaine hydrochloride and adrenaline (epinephrine) solution for injection 1:100,000. Articaine hydrochloride and adrenaline (epinephrine) solution for injection 1:200,000.
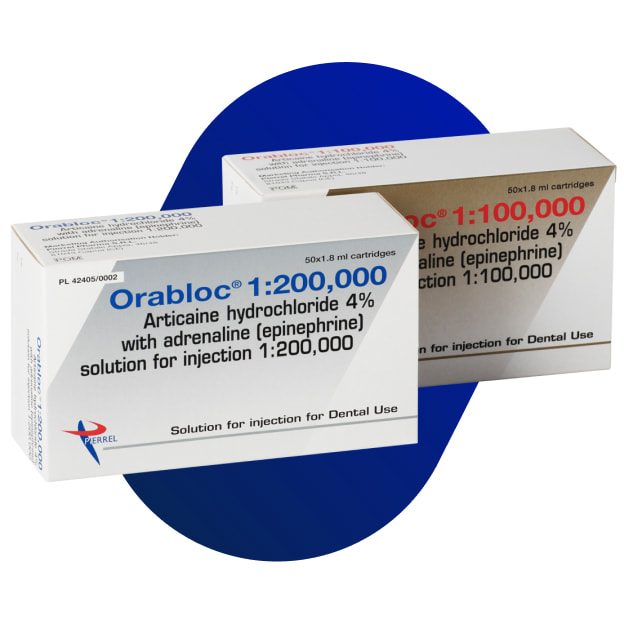


1 ml solution for injection contains 40 mg articaine hydrochloride and 0.01 mg adrenaline (epinephrine) as adrenaline tartrate.
One cartridge of 1.8 ml of solution for injection contains 72 mg articaine hydrochloride and 0.018 mg adrenaline (epinephrine) as adrenaline tartrate.
Excipients with known effect:
Contains sodium metabisulphite (E223) 0.5 mg/ml.
For the full list of excipients, see section 6.1.
See more – Orabloc 1:100,000 SPC – Orabloc 1:200,000 SPC
See more – Orabloc 1:100,000 SPC – Orabloc 1:200,000 SPC
Therapeutic indications
Orabloc is indicated in adults, adolescents and children of 4 years and older for local anaesthesia (infiltration and nerve-block anaesthesia) in dentistry:
See more – Orabloc 1:100,000 SPC – Orabloc 1:200,000 SPC
Pharmacodynamic properties
Pharmacotherapeutic group: Anaesthetics, local; Amides.
ATC code: N01BB58
Orabloc is an acid amide-type local anaesthetic used for terminal and nerve-block anaesthesia in dentistry. It is fast-acting (latency time 1-3 min) with a potent analgesic effect and good tissue tolerability.
The duration of effective anaesthesia is about 45 min for Orabloc 0.005 mg/ml and about 75 min for Orabloc 0.01 mg/ml.
The mechanism of action of articaine is assumed to be based on inhibition of conduction in nerve fibres, due to blockade of voltage-dependent Na+ channels in the cell membrane.
Its extremely low adrenaline (epinephrine) concentration and high intensity of action make Orabloc 0.005 mg/ml suitable for use in patients with cardiovascular diseases.
Paediatric population
In children 3.5 to 16 years old, clinical studies including up to 210 patients, have shown that 4% articaine + 0.005 mg/ml adrenaline (epinephrine) at doses up to 5 mg/kg and 4% articaine + 0.010 mg/ml adrenaline (epinephrine) at doses up to 7 mg/kg provided successful local anaesthesia, if given by (mandibular) infiltration or (maxillary) nerve block. The anaesthesia duration was similar for all age groups and depended on the volume administered.
See more – Orabloc 1:100,000 SPC – Orabloc 1:200,000 SPC
List of excipients
Sodium chloride
Sodium metabisulfite (E223)
Hydrochloric acid 2% (for pH adjustment)
Water for injection
Incompatibilities
Not applicable.
Shelf life
2 years
Special precautions for storage
Do not store above 25 °C. Store in the original package in order to protect from light.
Nature and contents of container
Clear glass cartridges (Type I) closed at one end with a bromobutylic rubber plunger and at the other with an aluminium cap and rubber seal.
The cartidge is available in different packages:
The cartridges are packaged in PVC blisters (10 cartridges/blister); the blisters are packaged in a cardboard box containing 5 x 10 or 10 x 10 cartridges.
Each cartridge is assembled in a plastic injector; each injector containing a cartridge is placed in a sealed blister; the injectors are packaged in a cardboard box together with Instructions for use of the injector: 50 or 100 units per commercial pack.
Not all pack sizes may be marketed.
Special precautions for disposal
As for any cartridge, the rubber seal (diaphragm) will be disinfected just before use with either pharmaceutical grade ethyl alcohol (70%) or pharmaceutical grade Isopropyl alcohol (90%).
The cartridges must not be immersed in the above solutions.
Do not mix the injectable solution with other products in the same syringe.
Any unused solution or waste material should be disposed of in accordance with local requirements.
See more – Orabloc 1:100,000 SPC – Orabloc 1:200,000 SPC
Pierrel Pharma S.R.L.
Strada Statale Appia, 46/48 – 81043 Capua (CE)
Italy
Date of first authorisation: 11/06/2013
Date of latest renewal: 03/05/2018
See more – Orabloc 1:100,000 SPC – Orabloc 1:200,000 SPC

https://www.dentalsky.com/
https://www.benco.com/

https://www.darbydental.com/

https://www.dcdental.com/
https://www.henryschein.com/us-en/dental/Default.aspx?did=dental

https://midwaydental.com/

https://www.pattersoncompanies.com/home/default.aspx

https://www.safcodental.com/

Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex.

Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex.
Orabloc is also sold by many other US and Canadian distributors, please check with your preferred distributor.
| Cookie | Durata | Descrizione |
|---|---|---|
| li_gc | 2 years | No description |
| wp-wpml_current_language | session | No description available. |
| Cookie | Durata | Descrizione |
|---|---|---|
| bcookie | 2 years | LinkedIn sets this cookie from LinkedIn share buttons and ad tags to recognize browser ID. |
| bscookie | 2 years | LinkedIn sets this cookie to store performed actions on the website. |
| lang | session | LinkedIn sets this cookie to remember a user's language setting. |
| lidc | 1 day | LinkedIn sets the lidc cookie to facilitate data center selection. |
| Cookie | Durata | Descrizione |
|---|---|---|
| cookielawinfo-checkbox-advertisement | 1 year | Set by the GDPR Cookie Consent plugin, this cookie is used to record the user consent for the cookies in the "Advertisement" category . |
| cookielawinfo-checkbox-analytics | 11 months |
Questo cookie è impostato dal plug-in GDPR Cookie Consent. Il cookie viene utilizzato per memorizzare il consenso dell'utente per i cookie nella categoria "Analytics".
|
| cookielawinfo-checkbox-functional | 11 months | Il cookie è impostato dal consenso cookie GDPR per registrare il consenso dell'utente per i cookie nella categoria "Funzionali". |
| cookielawinfo-checkbox-necessary | 11 months | Questo cookie è impostato dal plug-in GDPR Cookie Consent. I cookie vengono utilizzati per memorizzare il consenso dell'utente per i cookie nella categoria "Necessari". |
| cookielawinfo-checkbox-others | 11 months | Questo cookie è impostato dal plug-in GDPR Cookie Consent. Il cookie viene utilizzato per memorizzare il consenso dell'utente per i cookie nella categoria "Altri. |
| cookielawinfo-checkbox-performance | 11 months | Questo cookie è impostato dal plug-in GDPR Cookie Consent. Il cookie viene utilizzato per memorizzare il consenso dell'utente per i cookie nella categoria "Prestazioni".
|
| CookieLawInfoConsent | 1 year | Records the default button state of the corresponding category & the status of CCPA. It works only in coordination with the primary cookie. |
| elementor | never | This cookie is used by the website's WordPress theme. It allows the website owner to implement or change the website's content in real-time. |
| JSESSIONID | session | The JSESSIONID cookie is used by New Relic to store a session identifier so that New Relic can monitor session counts for an application. |
| viewed_cookie_policy | 11 months | Il cookie è impostato dal plug-in GDPR Cookie Consent e viene utilizzato per memorizzare se l'utente ha acconsentito o meno all'uso dei cookie. Non memorizza alcun dato personale.
|
| Cookie | Durata | Descrizione |
|---|---|---|
| _ga | 2 years | The _ga cookie, installed by Google Analytics, calculates visitor, session and campaign data and also keeps track of site usage for the site's analytics report. The cookie stores information anonymously and assigns a randomly generated number to recognize unique visitors. |
| _ga_19T11805C2 | 2 years | This cookie is installed by Google Analytics. |
| _ga_devsite | 2 years | This cookie is installed by Google Analytics. |

